The Problem
One of the most serious issues facing health authorities around the world is the problem of counterfeit or imitation pharmaceutical products, products that are produced and sold with the intent to deceptively represent their origin, authenticity, or efficacy. Many of these products do not contain any active ingredients, but instead contain inert substances that do not provide any treatment benefits to the patient. The World Health Organization estimates that 10% of all circulated medicines are counterfeit.
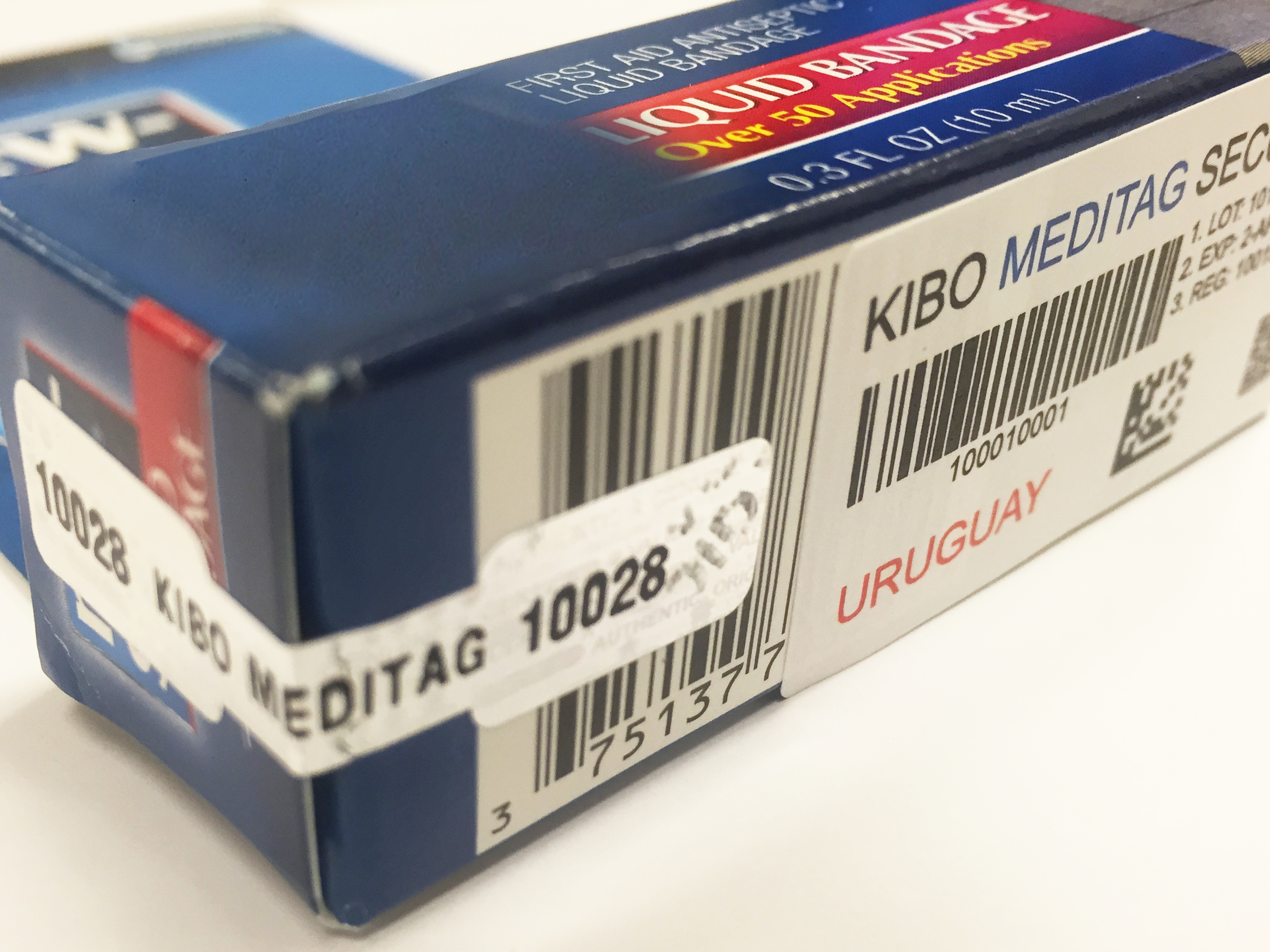
The counterfeit process can occur anywhere in the supply chain, from manufacture to delivery of the medicine to the end consumer. KIBO’s MediTag program effectively prevents faked and counterfeit medicines from entering the nation’s market.
The Technology
The MediTag program is based on the most advanced security methods and technologies. Legitimate pharmaceutical products are identified and then sealed with special self-destructive, tamper-proof MediTag labels. The security ink used to print the MediTag labels contains taggants, microscopic identification particles encoded with unique numeric sequences that quickly and reliably authenticate goods. Additional cutting-edge technologies, such as RFID tags and security barcoding, permit an even higher level of pharmaceutical security.
The MediTag Solution
KIBO’s MediTag program ensures that only those pharmaceutical products certified by the National Drug Authority are sold in the country.
- KIBO creates for the Government a database of all approved drugs with valid registrations and their authorized manufacturers and importers.
- KIBO produces unique, high-security, tamper-proof labels specifically coded to identify all government approved medicines.
- The Government issues an order requiring drug importers and domestic manufacturers to affix the MediTag security labels onto their products destined for the national market, thus securing their contents.
- KIBO trains government officials to operate the special scanners needed to identify and decode the MediTag security labels.
- Random inspections are carried out to confirm the authenticity of medicines at various points in the distribution process, including warehouses, pharmacies, and clinics. Location selection is determined by risk-analysis algorithms that KIBO provides to the Government.
- The information on the MediTag labels is compared to the packaged drugs to ensure consistency and transparency. Pharmaceuticals that fail the inspection process are confiscated and destroyed.
- Mandatory indemnification policies ensure that importers and manufacturers are held accountable for counterfeit and adulterated pharmaceuticals. Each must certify that their drugs meet or exceed the nationally established standards and provide samples and a sworn declaration on the product’s active and inactive ingredients to participate in the MediTag program.
- If it is necessary to confirm the composition of a drug sample, KIBO will perform an analysis of the medicine and its composition.
- KIBO assists the Government in implementing a public awareness campaign regarding the MediTag seals and the dangers of consuming unauthorized medicines.